ADAPT Clinical Trial LMS
Empowering Investigators and Sponsors through a custom-tailored Learning Management System
Bringing a medical device to market takes more than innovation. It requires precision, compliance, and confidence at every stage of the clinical journey. Ensuring every investigator and site team is properly trained and aligned is essential to trial success and regulatory readiness.
This customizable solution empowers your organization to deliver, track, and document each phase of investigator and site training, from protocol development through post-approval education. Built for consistency and accountability, it provides the structure and oversight needed to streamline training, strengthen compliance, and accelerate your path to market.
ADAPT LMS supports your customer education and sales team training needs. Tailored for life science organizations, ADAPT LMS offers your learners exactly what they need when and where they need it. Our learning management system offers flexibility to scale and customize to your specific needs.
Streamlined Study Training
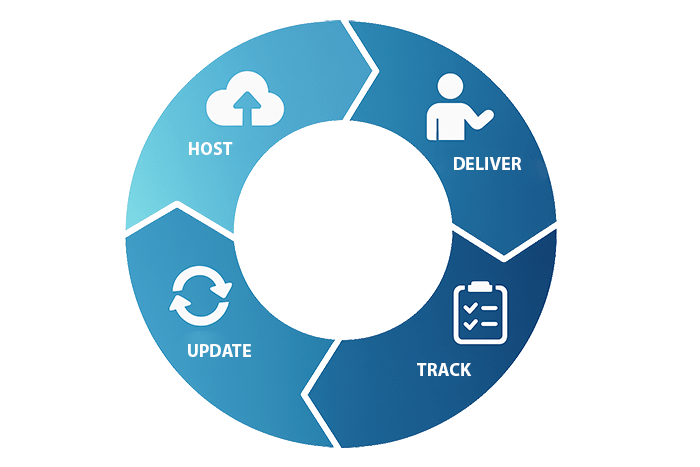
ADAPT centralizes every element of your clinical training program into one unified, validated system. Each phase, Host, Deliver, Track, and Update, is designed for regulatory compliance, version control, and study readiness.
Performance Gains for Every Stakeholder
You gain acceleration of study activation while reducing the training burden and delivering measurable outcomes for investigators, coordinators, and sponsors alike.
For Sites
For Sponsors
Built for Compliance and Validation
Your custom LMS is developed to meet the highest regulatory standards, aligning with FDA and GCP requirements, ensuring confidence from audit to inspection.
